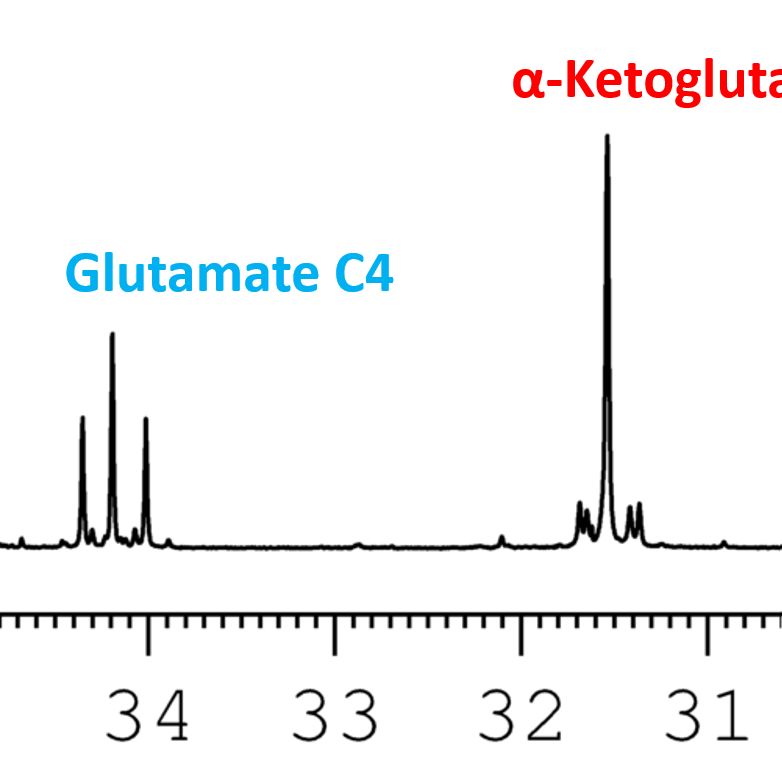
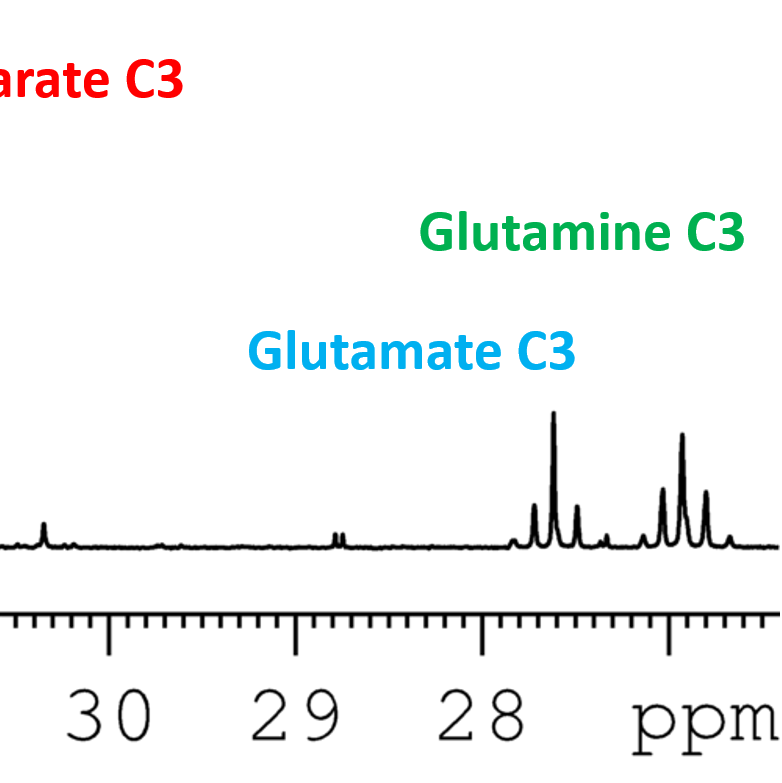
Below is an interactive tools that lets you select multiple metabolites and compare their 13C chemical shifts. If you prefer downloading the tables as pdfs, you will find links at the bottom of the page in the downloads section.
To the best of our knowledge these chemical shift values are accurate. However, because chemical shifts depend on pH, osmolality, and numerous other factors, these values should serve as guides, but should not be considered as standards.
A full list of references is provided below. Each chemical shift value has a reference denoted (a) through (w).
Currently works best in Google Chrome, you might experience some visual glitches in other browsers.
Create your own 13C chemical shift table
Select your metabolite(s) of intererest sorted by alphabet and/or by pre-defined groupings:
| 13C | ppm |
|---|---|
| C1 (d) | 182.1 |
| C1 (g) | 182.6 |
| C2 (d) | 27.3 |
| C2 (g) | 24.5 |
| C2 (w) | 24.6 |
| 13C | ppm |
|---|---|
| C1 (e) | 175.0 |
| C1 (d) | 175.5 |
| C1 (g) | 175.7 |
| C2 (d) | 54.2 |
| C2 (g) | 54.5 |
| C3 (d) | 211.0 |
| C3 (g) | 210.9 |
| C4 (d) | 30.5 |
| C4 (g) | 30.7 |
| 13C | ppm |
|---|---|
| C1 (g) | 30.5 |
| 13C | ppm |
|---|---|
| CH3 (d) | 21.5 |
| N(CH3) (d) | 54.7 |
| 13C | ppm |
|---|---|
| C1 (d) | 176.3 |
| C1 (g) | 176.7 |
| C2 (w) | 51.5 |
| C2 (g) | 51.7 |
| C2 (d) | 51.9 |
| C3 (d,g) | 17.3 |
| C3 (w) | 17.4 |
| 13C | ppm |
|---|---|
| C2 (w) | 34.8 |
| C3 (w) | 37.8 |
| 13C | ppm |
|---|---|
| C2 (w) | 55.8 |
| C3 (w) | 28.9 |
| C4 (w) | 25.5 |
| C5 (w) | 41.8 |
| C6 (w) | 157.7 |
| 13C | ppm |
|---|---|
| C1 (d) | 175.0 |
| C1 (g) | 175.3 |
| C2 (d) | 52.7 |
| C2 (g) | 53.4 |
| C3 (d) | 37.4 |
| C3 (g) | 37.8 | C4 (d) | 178.3 |
| C4 (g) | 178.5 |
| 13C | ppm |
|---|---|
| C1 (e) | 160.0 |
| C1 (d) | 160.8 |
| C1 (g) | 161.8 |
| 13C | ppm |
|---|---|
| C1 (e) | 184.0 |
| C3 (e) | 19.4 |
| 13C | ppm |
|---|---|
| C1/C5 (j) | 174.2 |
| C1/C5 (g) | 179.5 |
| C1/C5 (d) | 180.0 |
| C2/C4 (j) | 44.1 |
| C2/C4 (d) | 46.6 |
| C2/C4 (g) | 46.8 |
| C3 (j) | 74.2 |
| C3 (g) | 76.0 |
| C3 (d) | 76.1 |
| C6 (j) | 177.5 |
| C6 (g) | 182.3 |
| C6 (d) | 186.7 |
phosphoglycerate
| 13C | ppm |
|---|---|
| C1 (g) | 174.5 |
| C2 (g) | 78.6 |
| C3 (g) | 70.1 |
| 13C | ppm |
|---|---|
| C3 (w) | 37.8 |
| C2 (w) | 34.8 |
| C3 (w) | 37.8 |
| 13C | ppm |
|---|---|
| C1 (g) | 65.1 |
| C2 (g) | 96.0 |
| C3 (g) | 67.0 |
| 13C | ppm |
|---|---|
| C1 (g) | 66.5 |
| C2 (g) | 212.6 |
| C3 (g) | 68.2 |
| 13C | ppm |
|---|---|
| Ref | 67.4 |
| 13C | ppm |
|---|---|
| C3 (d) | 52.0 |
| 13C | ppm |
|---|---|
| C2 (d) | 58.2.0 |
| 13C | ppm |
|---|---|
| C1 (g) | 58.6 |
| C2 (j) | 17.4 |
| C2 (g) | 18.0 |
| 13C | ppm |
|---|---|
| C1 (g) | 83.2 |
| 13C | ppm |
|---|---|
| C1 (g) | 171.4 |
| C1 (w) | 172.2 |
| 13C | ppm |
|---|---|
| C1 (g) | 65.4 |
| C2 (g) | 105.9 |
| C3 (g) | 82.6 |
| C4 (g) | 77.8 |
| C5 (g) | 82.9 |
| C6 (h) | 64.4 |
| 13C | ppm |
|---|---|
| C1 (g) | 66.9 |
| C2 (g) | 102.0 |
| C3 (g) | 76.9 |
| C4 (g) | 75.1 |
| C5 (g) | 80.6 |
| C6 (h) | 65.3 |
| 13C | ppm |
|---|---|
| C1 (g) | 63.8 |
| C2 (g) | 105.3 |
| C3 (g) | 89.6 |
| C4 (g) | 76.9 |
| C5 (g) | 81.4 |
| C6 (h) | 64.5 |
| 13C | ppm |
|---|---|
| C1 (g) | 63.8 |
| C2 (g) | 102.5 |
| C3 (g) | 76.2 |
| C4 (g) | 75.4 |
| C5 (g) | 80.9 |
| C6 (h) | 65.3 |
| 13C | ppm |
|---|---|
| C1/C1' (d) | 175.3 |
| C1/C1' (g) | 176.6 |
| C2/C2' (d) | 136 |
| C2/C2' (g) | 136.4 |
| 13C | ppm |
|---|---|
| C2 (w) | 35.4 |
| C3 (w) | 24.6 |
| C4 (w) | 40.4 |
| 13C | ppm |
|---|---|
| C1 (h) | 180.0 |
| C2 (h) | 75.9 |
| C3 (h) | 72.3 |
| C4 (h) | 75.0 |
| C5 (h) | 73.2 |
| C6 (h) | 64.3 |
| 13C | ppm |
|---|---|
| C1 (g) | 93.2 |
| C6 (g) | 63.6 |
| 13C | ppm |
|---|---|
| C1 (w) | 97.0 |
| C6 (j) | 63.6 |
| 13C | ppm |
|---|---|
| C1 (w) | 92.9 |
| C1 (c) | 93.1 |
| C1 (g) | 93.2 |
| C2 (w) | 72.3 |
| C2 (d,g) | 72.5 |
| C3 (e) | 73.2 |
| C3 (d) | 73.8 |
| C3 (g) | 73.9 |
| C4 (e) | 70.1 |
| C4 (c,g) | 72.7 | C5 (w) | 72.3 |
| C5 (c) | 72.5 |
| C5 (g) | 72.6 |
| C6 (c) | 61.7 |
| C6 (g) | 61.8 |
| 13C | ppm |
|---|---|
| C1 (w) | 96.8 |
| C1 (c,g) | 97.0 |
| C2 (w) | 75.1 |
| C2 (c) | 75.2 |
| C2 (g) | 75.3 |
| C3 (e) | 76.1 |
| C3 (c) | 77.0 |
| C4 (e) | 70.1 |
| C4 (c,g) | 70.7 | C5 (c,w) | 76.8 |
| C5 (g) | 76.9 |
| C6 (c,g) | 61.8 |
| 13C | ppm |
|---|---|
| C1 (e) | 174.7 |
| C1 (d) | 175.1 |
| C2 (d) | 55.5 |
| C2 (w) | 55.8 |
| C3 (d) | 27.6 |
| C3 (w) | 28.1 |
| C4 (d) | 34.2 |
| C4 (w) | 34.7 |
| C5 (e) | 181.4 |
| C5 (d) | 181.8 |
| 13C | ppm |
|---|---|
| C1 (e) | 174.2 |
| C1 (d) | 174.8 |
| C2 (d) | 55.2 |
| C2 (w) | 55.4 |
| C3 (d) | 27.2 |
| C3 (w) | 28.1 |
| C4 (d) | 31.8 |
| C4 (w) | 32.0 |
| C5 (e) | 177.8 |
| C5 (d) | 178.5 |
| 13C | ppm |
|---|---|
| C1 (g) | 91.3 |
| C2 (g) | 74.9 |
| C3 (g) | 66.0 |
| 13C | ppm |
|---|---|
| C1 (g) | 63.4 |
| C1 (w) | 63.5 |
| C2 (g) | 72.4 |
| C3 (g) | 65.8 |
| C3 (w) | 66.0 |
| 13C | ppm |
|---|---|
| C1,C3 (f) | 62.6 |
| C1,C3 (d) | 63.5 |
| C1,C3 (j) | 63.6 |
| C2 (f) | 69.7 |
| C2 (d) | 73.0 |
| C2 (g) | 73.3 |
| 13C | ppm |
|---|---|
| C1 (g) | 173.5 |
| C2 (w) | 42.6 |
| C2 (j) | 42.7 |
| 13C | ppm |
|---|---|
| C1 (c) | 100.5 |
| C3 (c) | 74.0 |
| C4 (c) | 77.7 |
| C6 (c) | 61.4 |
| 13C | ppm |
|---|---|
| C1 (c) | 99.2 |
| C3 (c) | 73.5 |
| C4 (c) | 70.0 |
| C6 (c) | 67.9 |
| 13C | ppm |
|---|---|
| C1 (d) | 177.3 |
| C2 (d) | 60.3 |
| 13C | ppm |
|---|---|
| C1 (g) | 177.9 |
| C2 (g) | 89.3 |
| 13C | ppm |
|---|---|
| C2 (w) | 55.8 |
| C3 (w) | 29.7 |
| C6 (w) | 137.5 |
| C6 (w) | 117.5 |
butyrate
| 13C | ppm |
|---|---|
| C1 (e) | 180.5 |
| C1 (d) | 181.1 |
| C1 (g) | 181.2 |
| C2 (e) | 47.3 |
| C2 (d) | 47.6 |
| C3 (e) | 65.7 |
| C3 (d) | 66.6 |
| C3 (g) | 66.8 |
| C4 (d) | 22.7 |
| C4 (g) | 22.9 |
| 13C | ppm |
|---|---|
| N (w) | 56.7 |
| S (w) | 34.8 |
| 13C | ppm |
|---|---|
| C1,C3 (w) | 72.1 |
| C2 (w) | 73.3 |
| C4,C6 (w) | 73.6 |
| C5 (w) | 75.3 |
| 13C | ppm |
|---|---|
| C1,C5 (d) | 181.0 |
| C2 (d) | 38.5 |
| C3 (d) | 49.9 |
| C4 (d) | 74.6 |
| 13C | ppm |
|---|---|
| C2 (w) | 60.7 |
| C3 (w) | 37.0 |
| C4 (w) | 25.7 |
| CH3(4) (w) | 15.8 |
| CH3(5) (w) | 12.2 |
| 13C | ppm |
|---|---|
| C1 (d) | 170.7 |
| C1 (g) | 170.8 |
| C2 (d) | 206.3 |
| C2 (g) | 206.7 |
| C3 (d) | 31.4 |
| C3 (g) | 31.8 |
| C4 (d) | 36.7 |
| C4 (g) | 37.0 |
| C5 (d) | 182 |
| C5 (g) | 182.7 |
| 13C | ppm |
|---|---|
| C1 (d) | 183.2 |
| C1 (g) | 183.5 |
| C2 (d) | 69.4 |
| C2 (g) | 69.6 |
| C3 (d) | 21.0 |
| C3 (w) | 21.3 |
| 13C | ppm |
|---|---|
| C2 (w) | 55.3 |
| C3 (w) | 41.0 |
| C4 (w) | 25.3 |
| CH3 (w) | 22.2 |
| 13C | ppm |
|---|---|
| C2 (w) | 55.7 |
| C3 (w) | 31.2 |
| C4 (w) | 23.2 |
| C5 (w) | 27.3 |
| C6 (w) | 40.5 |
| 13C | ppm |
|---|---|
| C1 (d) | 182.1 |
| C2 (d) | 71.2 |
| C1 (g) | 71.7 |
| C3 (d) | 43.4 |
| C3 (g) | 43.9 |
| C4 (g) | 180.9 |
| C4 (d) | 181.6 |
| 13C | ppm |
|---|---|
| C2 (w) | 31.4 |
| C3 (w) | 55.1 |
| C4 (w) | 30.0 |
| 13C | ppm |
|---|---|
| C2 (g) | 140.7 |
| C3 (g) | 134.7 |
| C4 (g) | 148.6 |
| C5 (g) | 129.6 |
| C6 (g) | 143.4 |
| C7 (g) | 166.2 |
| 13C | ppm |
|---|---|
| C2 (g) | 139.2 |
| C3 (g) | 101.3 |
| C4 (g) | 22.9 |
| C5 (g) | 106.2 |
| C6 (g) | 125.1 |
| C7 (g) | 173.6 |
| 13C | ppm |
|---|---|
| C2 (g) | 148.3 |
| C3 (g) | 129.7 |
| C4 (g) | 137.0 |
| C5 (g) | 125.0 |
| C6 (g) | 152.5 |
| C7 (g) | 170.8 |
| 13C | ppm |
|---|---|
| C2 (g) | 149.9 |
| C3 (g) | 133.2 |
| C4 (g) | 138.6 |
| C5 (g) | 124.8 |
| C6 (g) | 151.2 |
| C7 (g) | 173.9 |
| 13C | ppm |
|---|---|
| C1 (g) | 178.2 |
| C2 (g) | 99.5 |
| C3 (g) | 50.0 |
| C4 (g) | 181.9 |
| 13C | ppm |
|---|---|
| C1 (g) | 169.3 |
| C2 (g) | 201.5 |
| C3 (g) | 50.2 |
| C4 (g) | 175.9 |
| 13C | ppm |
|---|---|
| C1 (g) | 175.5 |
| C2 (g) | 204.7 |
| C3 (g) | 50 |
| C4 (g) | 169.4 |
| 13C | ppm |
|---|---|
| C2 (w) | 57.2 |
| C3 (w) | 37.7 |
| C4 (w) | 136.8 |
| C5 (w) | 130.4 |
| C6 (w) | 130.0 |
| C7 (w) | 128.6 |
| 13C | ppm |
|---|---|
| N (w) | 67.6 |
| O (w) | 59.1 |
| 13C | ppm |
|---|---|
| CH2 (w) | 55.1 |
| CH3 (w) | 38.1 |
pyruvate
| 13C | ppm |
|---|---|
| C1 (g) | 174.2 |
| C2 (g) | 152.7 |
| C3 (g) | 102.1 |
amine
| 13C | ppm |
|---|---|
| N (w) | 41.8 |
| O (w) | 61.5 |
glycerate
| 13C | ppm |
|---|---|
| C1 (g) | 179.0 |
| C2 (g) | 77.4 |
| C3 (g) | 65.7 |
| 13C | ppm |
|---|---|
| C2 (w) | 62.3 |
| C3 (w) | 30.0 |
| C4 (w) | 25.0 |
| C5 (w) | 47.4 |
| 13C | ppm |
|---|---|
| C1 (d) | 185.4 |
| C2 (d) | 31.5 |
| C3 (d) | 11.0 |
carnitine
| 13C | ppm |
|---|---|
| C1 (w) | 176.8 |
| C3 (w) | 9.0 |
| 13C | ppm |
|---|---|
| C2 (w) | 59.1 |
| C3 (w) | 26.3 |
| C4 (w) | 30.9 |
| 13C | ppm |
|---|---|
| C1 (g) | 177.6 |
| C2 (g) | 93.1 |
| C3 (d) | 26.3 |
| C3 (g) | 26.7 |
| 13C | ppm |
|---|---|
| C1 (g) | 169.3 |
| C2 (g) | 205.4 |
| C3 (g) | 27.6 |
| 13C | ppm |
|---|---|
| C1 (g) | 171.1 |
| C2 (g) | 205.8 |
| C3 (g) | 27.3 |
| 13C | ppm |
|---|---|
| C1 (g) | 173.5 |
| C2 (w) | 57.4 |
| C2 (g) | 57.5 |
| C3 (g) | 61.4 |
| C3 (w) | 61.6 |
| 13C | ppm |
|---|---|
| C1 (g) | 63.6 |
| C2 (g) | 72.2 |
| C3 (g) | 70.8 |
| C4 (g) | 72.1 |
| C5 (g) | 74.1 |
| C6 (g) | 64.0 |
| 13C | ppm |
|---|---|
| C1,C1' (d) | 182.8 |
| C1,C1' (g) | 183.4 |
| C2,C2' (d) | 35.0 |
| C2,C2' (g) | 35.3 |
| 13C | ppm |
|---|---|
| S C1 (w) | 49.0 |
| S C1 (pH 6.5) (a) | 48.4 |
| S C2 (w) | 36.6 |
| S C2 (pH 6.5) (a) | 36.5 |
| 13C | ppm |
|---|---|
| C2 (w) | 61.5 |
| C3 (w) | 67.3 |
| C4 (w) | 20.7 |
| 13C | ppm |
|---|---|
| C1 (j) | 94.0 |
| C2 (j) | 94.4 |
| C2 (j) | 72.2 |
| C2 (j) | 72.3 |
| C3 (j) | 73.5 |
| C2 (j) | 73.7 |
| C4 (j) | 70.6 |
| C2 (j) | 70.9 |
| C5 (j) | 73.0 |
| C6 (j) | 61.5 |
| C6 (j) | 61.7 |
| 13C | ppm |
|---|---|
| C2 (w) | 56.0 |
| C3 (w) | 27.6 |
| C5 (w) | 126.0 |
| C8 (w) | 112.9 |
| C9 (w) | 123.0 |
| C10 (w) | 120.3 |
| C11 (w) | 119.4 |
| 13C | ppm |
|---|---|
| C2 (w) | 57.4 |
| C3 (w) | 36.7 |
| C4 (w) | 156.2 |
| C5 (w) | 131.8 |
| C6 (w) | 116.8 |
| C3 (w) | 127.6 |
| 13C | ppm |
|---|---|
| C2 (w) | 61.6 |
| C3 (w) | 30.3 |
| CH3 (w) | 19.2 |
It is also possible to download the 13C chemical shift table sorted by either metabolite name or by chemical shift:
Each value in the table above is assigned a reference (a) through (w), which are shown here:
| (a) | Chance EM, Seeholzer SH, Kobayahsi K, Williamson JR. J Biol Chem 1983, 258, 12785-13794. | |
| (b) | Cohen SM. J Biol Chem 1983, 14294-14308. | |
| (c) | Walker TE, Han CH, Kollman VH, London RE, Matwiyoff NA. J Biol Chem 1982, 257, 1189-1195. | |
| (d) | Samples dissolves in 2H2O, pH 7-7.4 °C. Dioxane at 67.4ppm used as internal standard. | |
| (e) | Pahl-Wostl C, Seelig J. Biochem 1986, 25, 6799-6807. | |
| (f) | Cohen SM. Annal NY Acad Sci 1987, 508, 109-129.M. | |
| (g) | London RE. Prog NMR Spectros 1988, 20, 337-383. | |
| (h) | Horton D, Walaszek Z, Ekiel I. Carb Res 1983, 119, 263-268. | |
| (i) | Ramos ML, Caldeira MM, Gil VMS. Carb Res 1997, 304, 97-109. | |
| (j) | Obtained from unknown source(s). | |
| (k) | Kunnecke B, Kustermann E, Seelig J. Magn Reson Med 2000, 44, 556-562. | |
| (l) | Schroeder MA, Atherton HJ, Ball DR, Cole MA, Heather LC, Griffin JL, Clarke K, Radda GK, Tyler DJ. FASEB J 2009, 23, 2529-2538. | |
| (m) | Spectral database for organic compounds | |
| (n) | Ippel JH, Wijmeng SS, de Jong R, Heus HA, Hilbers CW, de Vroom E, van der Marel GA, van Boom JH. Magn Reson Chem 1996, 34 S156-S176. | |
| (o) | Kishore AI, Mayer MR, Prestegard JH. Nucleic Acids Res 2005, 33, e164 | |
| (p) | Measured using conditiions in ref (d). Chemical shift referenced to Pyr-Cl at 171ppm, 37°C. | |
| (q) | Samples dissolved in PBS, pH 7-7.4, 37°C, Chemical shift referenced to d-Gluconolactone-C1 at 173.8ppm. | |
| (r) | Micheli A, Tomassini A, Puccetti C, Valerio M, Peluso G, Tuccilo F, Calvani M, Manetti C, Conti F. Biochemie 2006, 88, 437-448. | |
| (s) | Gottlieb HE, Kotlyar V, Nudelman A. J Org Chem 1997, 62, 7512-7515. | |
| (t) | Benesi AJ, Falzone CJ, Banerjee S, Farber GK. Carb Res 1994, 258, 27-33. | |
| (u) | Drew KN, Zajicek J, Bondo G, Bose B, Serianni AS. Carb Res 1998, 307, 199-209. | |
| (w) | Obtained from unknown source(s). |
| Date | Version | Comments |
|---|---|---|
| 12/26/2018 | 1.1 | Removed functionlity and design of letter buttons that lead nowhere. Added change notes and reworked supporting info. |
| 12/20/2018 | 1.0 | Used all metabolites from PDF to create basic set |